Evolution M&A Analysis: China seeks food security with $43 billion bid for Syngenta
February 11, 2016
Evolution M&A Analysis: Evaluating Potential Acquisition Targets for Thermo Fisher Scientific
March 17, 2016
For our latest Data Visualisation project, the Evolution Global Team analysed historical new drug approval statistics and the current clinical trials pipeline to identify trends that highlight the rise and diversification of Biologics. From start-ups to blue chips, we have evaluated the pharmaceutical players involved in the ongoing research and development of Biologics in order to identify upcoming therapeutic categories with disruptive potential.
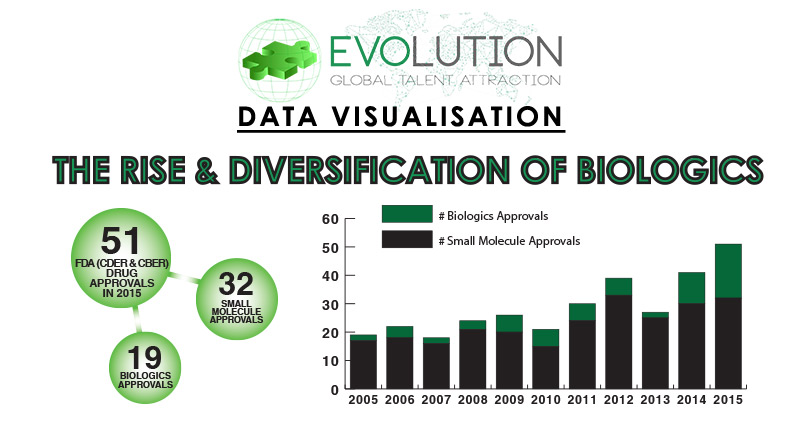
Chart 1: Small Molecule & Biologics FDA Approvals by Year
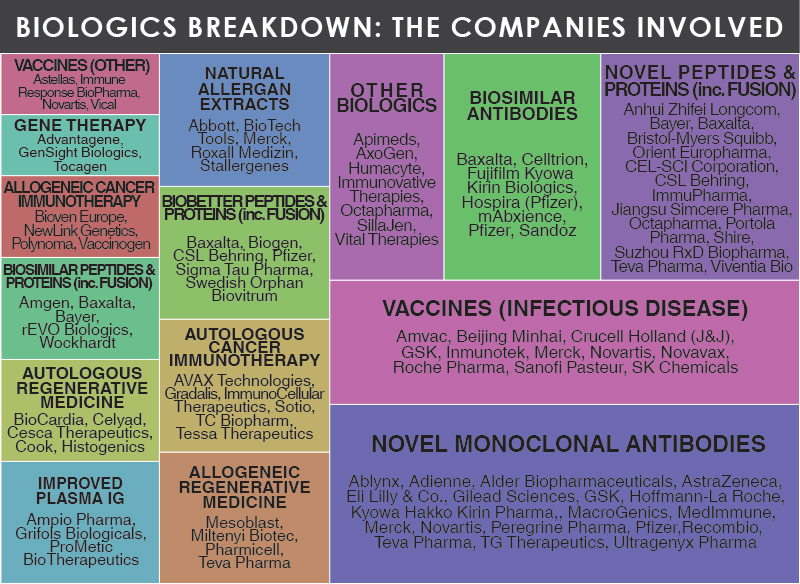
2015 saw 51 New Drug Approvals by the FDA (CDER & CBER), with 32 of these drugs classed as Small Molecules and 19 classed as Biologics. Chart 2 highlights divides these newly approved Biologics into eight categories, with the dominant class being Monoclonal Antibodies, accounting for 42% of approvals. Recombinant Peptides & Proteins accounted for 21% of approvals, whilst Biobetter Recombinant Peptides & Proteins ranked third with 11% of overall Biologics approvals. In 2015 a single Biologic was approved for each of the five other classes (Antibody Fragments, Oncolytic Viral Therapy, Recombinant Fusion Proteins, Recombinant Glycoproteins and Vaccines).
Chart 2: Biologics Approvals by Category (Hover for values)
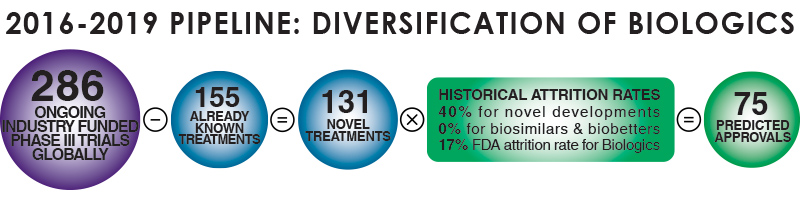
In-depth analysis of the Clinical Trials pipeline from 2016-2019 has identified 286 ongoing industry-funded Phase-III trials globally, 131 of which are classified as novel treatments. By applying historical attrition rates Dr. Frank Rinaldi, Director of Market Intelligence & Innovation at Evolution, has forecast that 75 of these novel treatments will be approved. Chart 3 splits the novel treatments into categories, highlighting the predicted number of approvals for each type of treatment.
Chart 3: Biologics Approvals Forecasting
“It’s clear that there are some exciting new developments ahead, and that the therapeutic class known as Biologics will continue to expand to include a wider use of cell therapy treatments, allogeneic and/or autologous,” notes Dr. Rinaldi. “The impact of cancer immunotherapy and regenerative medicine approaches will also be significant. For both regenerative medicine and cancer immunotherapy using autologous mechanisms, a further interest will be the ability to deliver treatment cost effectively in order to avoid failures similar to Dendreon’s prostate cancer vaccine.”
“Another area to watch out for is the expansion of the extrapolation concept for biosimilars,” notes Dr. Rinaldi. “Celltrion’s biosimilar version of Remicade is likely to be approved by the FDA to treat several or possibly all of the conditions J & J’s Remicade treats. It would be the first biosimilar to be approved in the U.S. for a number of different diseases at one step, overcoming the requirement for clinical trials for every use indication.”
“Last but not least are the new Monoclonals in development,” states Dr. Rinaldi. “Notable mAbs in development include Roche/Genentech’s Ocrelizumab for both relapsing/remitting and primary progressive multiple sclerosis, and Atezolizumab, which is in several phase 3 trials to treat a number of tumour types. If successful, both of these developments are forecast to be >$2B per annum blockbusters.”

As Biologics expand to include a wider use of cell therapy treatments, the cell becomes the product. Dr. Jason Beckwith, Managing Director at Evolution Global, anticipates that the industry is likely to experience a talent demand paradigm shift for enhanced expertise specific to complex Quality Control, Cell Characterisation, Regulatory & Logistics skill-sets. “As classical bioproduction shifts to different therapeutic areas, it is key that specialised talent providers are aware of their client strategy and understand their bespoke requirements,” asserts Dr. Beckwith. “A competent technical search provider will be an important industry partner to help provide the required differentiation for strategic success in this evolving dynamic market.”
You can download a PDF copy of our full Biologics infographic below:
Follow Evolution Global Talent Attraction on Twitter, Facebook and LinkedIN to keep up-to-date with news and trends from the biotechnology, biosciences, medical device, IT and Intellectual Property industries.