
Exploring the History & Viability of Xenotransplantation: Porcine Retroviruses, CRISPR Off-Target Effects, Immune Rejection & Regulatory Hurdles
August 22, 2017
2017 Global Therapeutic IPO Market: 121% YOY Increase in Funds Raised Despite Slow Q3
October 5, 2017[vc_row][vc_column][vc_column_text]
Gilead Sciences has ended its M&A moratorium with an $11.9B all-cash acquisition of CAR-T Cell Therapy pioneers Kite Pharma. Kite’s leading drug Axi-Cel is expected to receive FDA approval in November, and will likely go head-to-head with Novartis’ Kymriah which received FDA approval on 30th Aug 2017. Considering the fact that Gilead has historically focused on the treatment of viruses and tumours with small molecule drugs, the Kite acquisition represents a bold, high-risk strategic move into the field of cell therapy. In our latest M&A Analysis we explore the challenges Gilead are likely to face in the coming years.
The CAR-T Process
This type of treatment is predicated on the basis that the engineered expression of chimeric antigen receptors (CARs) on the surface of T-cells enables the redirection of T-cell specificity. Overall, early stage clinical trials utilising CAR-T cells for the treatment of patients with cancer have shown modest results, with the exception being the remarkable outcomes of several trials of CD19-targeted CAR-T cells in the treatment of patients with B-cell malignancies. These results have generated an amplified interest for this approach over the last couple of years. This is where Kite’s initial development KTE-C19 (axicabtagene ciloleucel, axi-cel) is positioned, and as such is on the cusp of the enthusiasm curve.
Given that the treatment is what is known as autologous, the supply chain aspects are complicated relative to ‘off the shelf’ small molecules and traditional biologics such as monoclonal antibodies. The steps required involve:
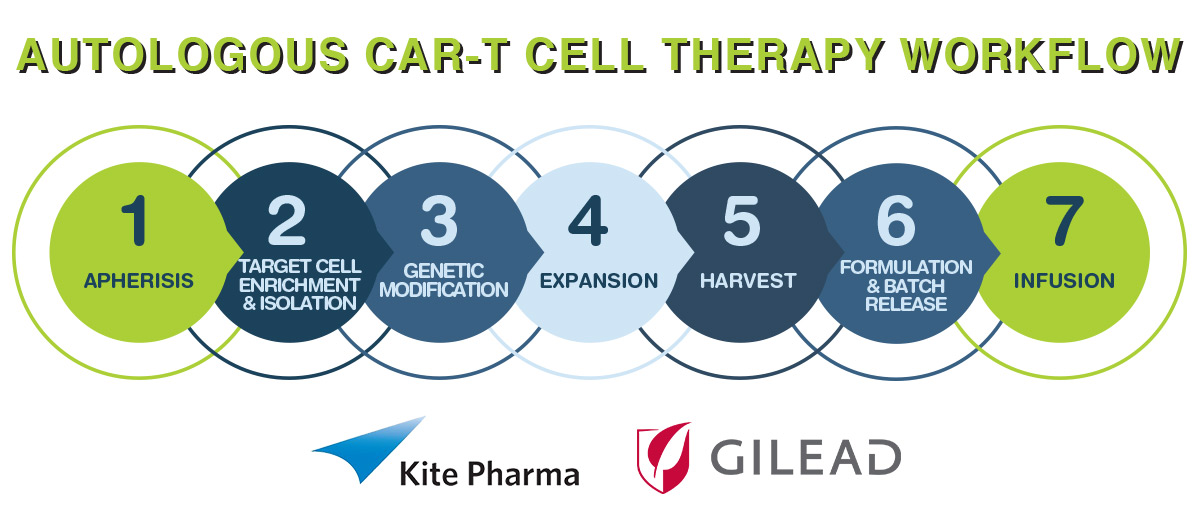
Supply Chain Challenges
The process for KTE-C19 takes approximately 14 to 16 days, starting from receipt of the patient’s white blood cells to a dedicated manufacturing facility through to the release of the engineered T-cells for transfusion back to the patient.
Critical to the concept is the participating supply chain that will conduct the apheresis and the final infusion. In this regard, Kite has been active in establishing relationships with top-tier hospitals that have experience with autologous T-cell therapies and that treat the largest number of patients with aggressive Non-Hodgkin’s Lymphoma (NHL).
Kite intends to establish sites and relationships directly in US and Europe, with the Company having already partnered with Fosun Pharma (JV FOSUNKite) in China and Daiichi-Sankyo in Japan. If clinically successful, Kite’s intention is to target an initial patient annual opportunity of 47,200 worldwide. Given that the current manufacturing set-up can process 4,000 patients annually, this would suggest that at least 12 sites will be required globally, although it should be noted that the setup is modular, and can be expanded if logistically feasible at a single geographic location.
It is envisaged that initial approval and launch of Axi-Cel will take place in the US in late 2017, with extension from there. From a patient volume perspective, it’s likely that China will eventually be the largest market.[/vc_column_text][vc_row_inner][vc_column_inner width=”1/6″][/vc_column_inner][vc_column_inner width=”2/3″][vc_column_text]
[/vc_column_text][dfd_spacer screen_wide_resolution=”1280″ screen_wide_spacer_size=”20″ screen_normal_resolution=”1024″ screen_tablet_resolution=”800″ screen_mobile_resolution=”480″ screen_normal_spacer_size=”19″ screen_tablet_spacer_size=”7″ screen_mobile_spacer_size=”5″][/vc_column_inner][vc_column_inner width=”1/6″][/vc_column_inner][/vc_row_inner][vc_column_text]
Exploring Kite’s Development Assets
Other important development assets in the Kite portfolio include KITE-718, a therapy targeting MAGE A3/A6 positive cancers, including non-small cell lung cancer (NSCLC) and bladder cancer. However, it should be noted that attempts so far to treat solid tumours with CAR-T approaches have had significant issues related to ‘on-target, off-tumour’ toxicity, and the modulation of the immunosuppressive tumour microenvironment.
Avoiding the Fate of Dendreon’s Provenge
Assuming that Axi-Cel will be approved this year, a major concern is whether the treatment will be as commercially unsuccessful as Dendreon’s Provenge. Despite the technical variation in the approach being considered (i.e. CART vs Dendritic cells) and the fact that the initial development is to treat NHL rather than Prostate cancer, the actual logistical processes and reimbursement requirements are likely to be similar. Despite being covered by Medicare, prostate cancer specialists did not favour the therapy. There were several reasons for this including:
1. Many did not believe that Provenge warranted the price tag of $93,000 in terms of the patient benefit. In other words, they did not accept the value-proposition. In contrast, Axi-cel appears to offer a manageable patient risk profile with some concerns.
2. Provenge was administered as three doses spread over two weeks. This required specialists to commit to a large cash outflow in a short period of time with the reimbursement process too slow to minimise cash flow challenges. In contrast, Axi-Cel is a single infusion. However, there is still a risk if claims are not processed in a timely manner. Part of the Provenge reimbursement problem was the novelty of the product, with payers slow to refund. Specialists who were supportive of Provenge in many cases grew weary of waiting for reimbursement.
3. Two small molecule drugs for castration-resistant prostate cancer, Xtandi (Astellas) and Zytiga (Janssen), were approved not long after Provenge. The advent of these lower cost treatment options with standard reimbursement channels could not have occurred at a worse time for Dendreon. In contrast, it’s important to note that current treatment regimens have efficacy in 50% to 60% of patients with Diffuse Large B-cell lymphoma (DLBCL), the most common type of lymphoma. The last major approval was in 2006, when the FDA approved rituximab for use in the first-line treatment of patients with DLBCL in combination with anthracycline-based chemotherapy regimens. Since then, no other agents have been approved for the treatment of DLBCL.
Provided that the reimbursement challenge with regards to cell-based immunotherapies is addressed by efficient frameworks for the reimbursement and a consideration that the price leveraged is fair, we expect physicians to prescribe these treatments if clinically successful. Ultimately the attraction will be based on comparative clinical effectiveness of the novel therapy vs. a relevant comparator in the given markets being targeted. This requires to be coupled to an attractive cost-utility based on absolute improvement in 1-year survival vs. standard of care.
CAR-T Competition
CAR-T cells as a treatment opportunity are being pursued by multiple companies including: Adaptimmune LLC, bluebird bio, Inc., Celgene Corporation, Immunocore Limited, Juno Therapeutics, Lion Biotechnologies, Novartis, Mustang Bio, Inc, Takara Bio Inc., ZIOPHARM Oncology, Inc, as well as several China-based companies. In addition, companies such as Cellectis are pursuing allogeneic T-cell products, including an anti-CD19 allogeneic T-cell product.
Of note is that Novartis and Juno Therapeutics, with the support of Celgene Corporation, are developing comparable versions of anti-CD19 CAR-T cell therapies. The attraction of the treatments is based on the fact that the therapy has previously shown enticing potential, attaining remission in patients for whom alternative interventions have failed. Of the two, the Novartis therapy now branded as Kymriah (tisagenlecleucel) for certain paediatric and young adult patients with a form of acute lymphoblastic leukaemia (ALL), was approved on 30th of August 2017 by the FDA. This is a historic approval by the FDA as it’s the first gene therapy approved in the US.
Novartis’ price point of $475,000 for one-time treatment suggests that they may well experience similar issues to Provenge. However, it is important to note that childhood ALL is rare, compared to Prostate cancer making little impact on the overall healthcare budget. Additionally, one is dealing with the emotive subject of treating seriously ill children and young adults. The price cited is 36% less than the value analysts were predicting. The justification is the need to recoup development costs and gain an adequate financial return, and the fact according to Novartis CEO that ‘the cost of goods, the cost of manufacturing these cells and processing is very, very high’.
Toxicity & Side Effects
The field is not without its challenges. In 2016, Juno Therapeutics reported one of the 68 patients in its relapse/refractory Acute Lymphoblastic Leukaemia (r/r ALL) trial being treated with JCAR015 died from cerebral oedema. In July, a further two patients died from the same condition and the trial was suspended. After appealing to the FDA, Juno investigators recommenced the trial, omitting an accompanying chemotherapy drug they suspected was responsible for the adverse reactions, only to have the study halted again after two more deaths from cerebral oedema in November. In March 2017, Juno discontinued JCAR015 in favour of JCAR017, which is hoped to be less toxic.
It should be noted that while the recently approved Kymriah has demonstrated noteworthy 82% remission rates. In a 2016 trial with 50 children and young adults with ALL, the treatment caused severe cytokine release syndrome (CRS) in nearly half of patients treated. To counteract this effect the FDA also on the 30th of August, approved Roche’s Actemra (tocilizumab) to treat CAR T-cell-induced severe or life-threatening CRS in patients 2 years of age or older. In clinical trials in patients treated with CAR-T cells, 69% of patients had complete resolution of CRS within two weeks following one or two doses of Actemra. It is hoped that this secondary intervention will address the CRS challenge.
Kite Pharma has also reported serious neurological side effects in one-third of its NHL patients and CRS in one-fifth, as well as two patient deaths, during a trial of its lead CD19-targeting candidate KTE-C19.
The toxicity concerns have resulted in the FDA proposing the creation of central databases to keep track of safety indications for CD19-targeting CAR-T cell therapies. The goal is to combine small data sets to enable identification of steps in development or administration that are linked to increased risks. Developments of so-called next generation treatments are being actively pursued with the aim of reducing toxicity. However, it’s likely that the first generation to be approved (Novartis, Kite) will require extensive monitoring to ensure toxicity is minimised.
In this regard, the FDA states that Kymriah is being approved with a risk evaluation and mitigation strategy (REMS), which contains elements to assure safe use (ETASU). The FDA mandates that hospitals and their related clinics are specially certified. Critical to the certification is the absolute assurance that personnel tasked with the prescribing, dispensing, or administering of Kymriah are trained to recognise and manage CRS and neurological events. Furthermore, the certified settings must have protocols in place to ensure that Kymriah is only given to patients after confirming that tocilizumab is accessible for immediate application if required. The REMS program also stipulates that patients be knowledgeable of the signs and symptoms of CRS and neurological toxicities that may follow infusion and of the importance of promptly returning to the treatment site if they develop fever or other adverse reactions. To further evaluate the long-term safety, Novartis is also required to conduct a post-marketing observational study involving patients treated with Kymriah.
Conclusions
Ultimately the question is why a company such as Gilead should engage in this area. Gilead’s portfolio and experience is largely in the treatment of viral diseases with small molecules drugs, most notably in treating AIDs/HIV and Hepatitis C, where Gilead has clear leadership. Gilead’s notable oncology asset is Zydelig for the treatment of chronic lymphocytic leukaemia (CLL), indolent B-cell non-Hodgkin’s lymphoma (NHL) and small lymphocytic lymphoma (SLL). This is a small molecule first in class PI3k inhibitor, used in combination with rituximab or ofatumumab. Relative to other pharma players, Gilead lacks significant experience in the disease indications being targeted and in the complexities of cell therapy.
Like all major pharma companies, Gilead has the strategic challenge of developing or acquiring new blockbuster treatments before its current revenue-producers diminish and/or their patent protection expires. Strategically addressing this concern has largely been one of establishing and maintaining a clear leadership position in a given category.
With the Kite acquisition, Gilead is embarking on a bold move to actively disrupt its own current business model. The recent Novartis Kymriah approval does represent at least the potential to gain approval with CART treatments is there, but what is not clear is the return. The outstanding success of treating childhood ALL which has seen increases in survival from 10% in the 1960s to 90% in 1990s, means Kymriah is only going to be considered for treating up to 500 to 600 patients in the USA annually. Resulting in a manageable $285M annual impact to the US healthcare reimbursement budget. NHL is a much more common condition, with Kite estimating an eligible patient population of 7,400 annually. At a similar pricing strategy, this represents a $3.5B annual impact to the US healthcare budget. This may be a value deemed too great given the likelihood of the additional costs of minimising toxicity, etc.
What can be stated with certainty is that Gilead has historically been extremely successful with M&A activity, having allocated $15 billion in acquisitions over the last decade with remarkable returns. They are also very effective at leveraging significant prices for their products, with Solvadi and Harvoni notable examples.
This year they have been under pressure from major shareholders & analysts to execute M&A activity in response to declining sales in the hepatitis C market. Diversification outside of antivirals has been promoted as providing benefits from an improving business mix outweighing potential near-term dilution.
Will Gilead’s acquisition of Kite prove successful in the long term? In many ways, this is a binary acquisition: if successful it will be another example of the highly lucrative Gilead M&A approach; if unsuccessful the acquisition will be written off as an impulsive reaction driven by shareholders who have backed the management into a corner and created a situation where action was carried out for short term reasons as opposed to longer term considerations.
Given that Gilead have a great track record in M&A and its due diligence is thorough, the company will have weighed the risks posed by the acquisition of Kite against the long and short term strategic benefits. Ultimately the answer to the question is only one that time will tell.[/vc_column_text][/vc_column][/vc_row][vc_row][vc_column width=”1/6″][/vc_column][vc_column width=”2/3″][vc_column_text]
Follow Evolution Global on LinkedIN, Twitter & Facebook to keep up-to-date with news and trends from the Biotechnology, Biopharma, Life Sciences & Informatics industries.
[/vc_column_text][/vc_column][vc_column width=”1/6″][/vc_column][/vc_row]



