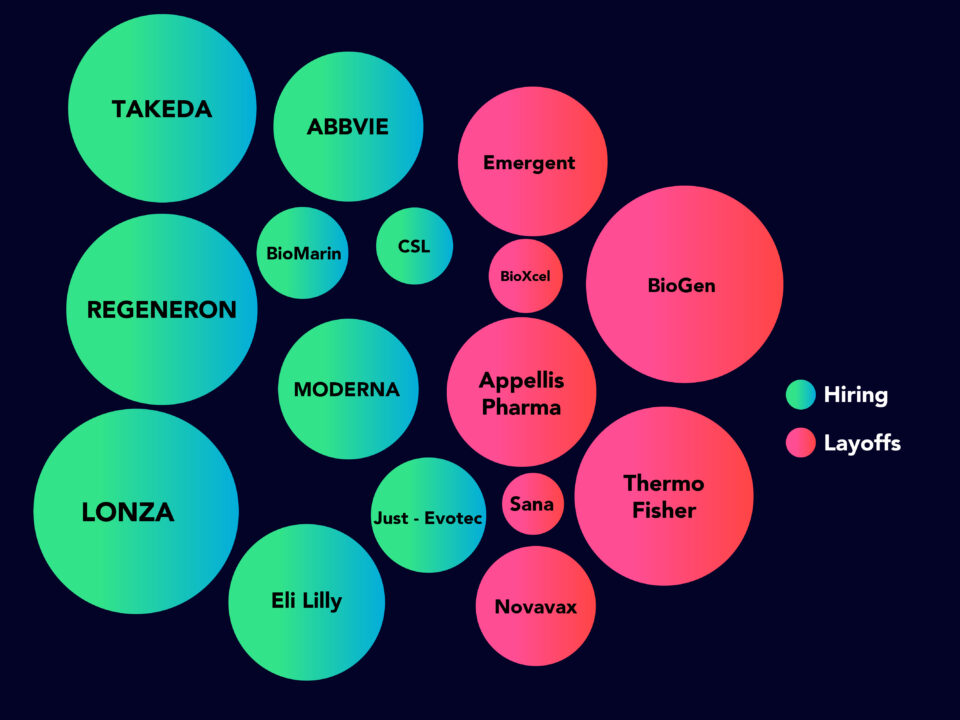
BioPharma – Positive Hiring News
September 6, 2023AI takes center stage at J.P. Morgan Healthcare Conference
2024 will continue to experience the introduction of artificial intelligence in the drug discovery sector, potentially disrupting the biopharma industry, as exemplified by Google parent Alphabet’s digital biotech company Isomorphic Labs announcement of two large deals worth nearly $3 billion signed with Eli Lilly and Novartis, announced recently at the JPM conference.
The AI boom has seen the rise of the “Magnificent 7,” a new grouping of mega-cap tech stocks comprised of the seven largest U.S.-listed companies—tech giants Amazon, Apple, Alphabet, Microsoft, Meta Platforms, Nvidia and Tesla, with a combined market value increase of almost 75% to $12 trillion, demonstrating their collective financial power.
The impact of AI for drug discovery is still largely unknown, as the public market valuation of the few AI-drug discovery companies is significantly down versus their peak price, and a large chunk of the high-value deals announced between native AI companies and large Pharmas are essentially based on future milestone payments which may never materialise.
2023 Deals
AI startups have been at the centre of a wave of deals driven by companies looking to enrich their drug discovery pipelines. In August 2023, Exelixis paid $80 million to license a small-molecule inhibitor of USP1 from Insilico Medicine, an AI biotech headquartered in Hong Kong. The deal grants Exelixis the right to develop and commercialize lead molecule ISM3091, aimed at a synthetic lethal target implicated in BRCA-mutated tumors, identified by Insilico’s generative AI engine.
In September 2023,, Merck KGaA signed separate deals with BenevolentAI and Exscientia worth more than $30 million up front and $1 billion in potential milestones. Merck selected three targets for each company to work on identifying small molecules uncovered by their respective AI discovery platforms. Also in September 2023, AI drug-discovery biotech Verge Genomics signed a four-year pact in neurodegenerative diseases with Alexion, wholly owned by UK-based AstraZeneca. Verge’s platform uses human tissue data combined with computational analysis to find drug targets. The biotech, which has already taken one ALS program into the clinic, is eligible for $840 million in milestones plus royalties.




Genesis Therapeutics, a biotech that uses generative and predictive AI technologies to design small-molecule drugs, raised $200 million to expand its pipeline. Its platform combines deep learning, molecular simulations and language models to generate molecules with high potency and selectivity. The technology trains on molecular simulations and wet-lab data to represent the physical interactions in protein–ligand complexes as 3D spatial graphs. This approach allows it to predict novel targets without the need for binding affinity data.
Generate Biomedicines, a startup set up by Flagship Pioneering in 2018 to use machine learning to identify antibodies, peptides and cell therapies, raised $273 million in a series C funding. It plans to take multiple new molecules into trials, adding to its preclinical and clinical pipeline of 17 programs across oncology, immunology and infectious disease.
AI and ML enhance efficiency in drug discovery
Selecting the best candidate molecule to progress from discovery to new chemical entity (NCE) is complex and time consuming. As a result, drug discovery teams are increasingly looking to improve the quality of their initial hit molecules to get a head start on this process. Drivers for this include that it is significantly harder to find novel molecules to bind to many drug targets of current interest, especially compared to twenty or thirty years ago. This has led to increased interest in evaluating many more molecules than previously, without significantly increasing wet chemistry and biology budgets.
AI and ML methods running on latest generation hardware can triage molecules at a scale that was unthinkable several years ago. Current practice contemplates ‘virtual library’ sizes of tens of billions of molecules; either in a ‘bottom up’ scenario where the library consists of molecules defined by a set of synthetic reactions and appropriate starting materials to form an ultra-large virtual library (ULVL), and/or a ‘top down’ scenario where the chemical space is defined by the assembly using a ‘generative AI’ of molecules from a ‘molecular soup’ of small chemical fragments. Each approach has strengths and weaknesses – generative AI, for instance, tends to suggest many molecules that are synthetically inaccessible or chemically unstable. Conversely, the ULVL contains predominantly synthetically accessible molecules, but has biased (and constrained) diversity, so does not explore as widely. In both cases, there is a large-scale computing challenge inherent in considering billions of options and reducing that down to a few tens to hundreds of molecules, which will then be made or acquired and tested against the biological target of interest.
Although the filtering process, at least for early drug discovery, can be (or must be) highly automated, in the later stages, when nearing candidate selection, the process naturally switches to human decision making, albeit in the context of the multi-factorial data generated throughout the discovery project to-date. The nature of the AI ‘support’ to the human scientist must therefore alter throughout the discovery process.
Overcoming limitations to embrace the future of AI
AI/ML techniques have already made a significant impact on drug discovery, offering advanced solutions to funnel potential candidates through the pipeline at speed. Although AI has the potential to solve other limiting steps in drug discovery, application of AI/ML systems is restricted by the data available, as significant amounts of information is required to train an AI model. The nature of the drug discovery process necessarily requires the implementation of strict data protection and privacy, which does unfortunately limit data sharing.
To really benefit from AI, the pharmaceutical industry must be more open to data sharing. Larger amounts of available data would significantly increase the range of problems to which AI/ML tools can be applied. Complex molecular molecular properties in the areas of absorption, distribution, metabolism or excretion (ADME) could be better predicted, accurately filtering out poor candidates and streamlining discovery.
Another limitation to date has been the relative scarcity of experts who are proficient in both drug discovery and AI, whom are necessary to ensure that the tools will be utilised efficiently to deliver accurate insights and to inform and accelerate drug discovery.
AI and ML methods running on latest generation hardware can triage molecules at a scale that was unthinkable several years ago. Current practice contemplates ‘virtual library’ sizes of tens of billions of molecules; either in a ‘bottom up’ scenario where the library consists of molecules defined by a set of synthetic reactions and appropriate starting materials to form an ultra-large virtual library (ULVL), and/or a ‘top down’ scenario where the chemical space is defined by the assembly using a ‘generative AI’ of molecules from a ‘molecular soup’ of small chemical fragments. Each approach has strengths and weaknesses – generative AI, for instance, tends to suggest many molecules that are synthetically inaccessible or chemically unstable. Conversely, the ULVL contains predominantly synthetically accessible molecules, but has biased (and constrained) diversity, so does not explore as widely. In both cases, there is a large-scale computing challenge inherent in considering billions of options and reducing that down to a few tens to hundreds of molecules, which will then be made or acquired and tested against the biological target of interest.
Although the filtering process, at least for early drug discovery, can be (or must be) highly automated, in the later stages, when nearing candidate selection, the process naturally switches to human decision making, albeit in the context of the multi-factorial data generated throughout the discovery project to-date. The nature of the AI ‘support’ to the human scientist must therefore alter throughout the discovery process.
The Future of AI in Drug Discovery
It is clear that AI can positively impact challenges encountered throughout the drug discovery process. Current limitations to adoption hinge on training ML models, with restricted access to data and strict confidentiality in the pharmaceutical industry forming a barrier. Interest in utilising AI is on the rise, however, for applications including approximation of quantum mechanics, generation of optimal synthetic routes for compounds, predicting protein structures, accurate prediction of FEP calculations, and scaling of docking. As more data is generated, ML tools could be better positioned to address these complex calculations.
Integration of AI/ML into drug discovery platforms has already led to considerable improvements, with collective efforts accelerating drug discovery rates, enhancing efficiency and reducing costs. AI/ML systems will continue to evolve and shape the pharmaceutical industry, significantly enhancing scale and accelerating candidate selection.

Evolution provides elite executive search & headhunting solutions for companies at the forefront of scientific and technological advancement. We provide bespoke recruitment solutions for our exclusive clients on a global level; providing a deeper service provision with value-added services.