Evolution Briefing: Novavax Announce New SVP Global Sales
July 7, 2020
Evolution Briefing: COVID-19 Vaccine August Update
August 11, 2020Introduction
Researchers worldwide are working around the clock to find a vaccine against SARS-CoV-2. Experts estimate that a fast-tracked vaccine development process could speed a successful candidate to market in approximately 12-18 months, providing the process from conception to market availability runs smoothly.
The pandemic has sparked many unprecedented partnerships between public and private companies in the pharmaceutical industry. An example lying in ‘Operation Warp Speed’ (OWS), a collaboration of several US federal government departments and its subagencies combining to tackle a single disease.
The US government plans to select 3 vaccine candidates to fund for Phase III trials under OWS:
- Moderna’s mRNA-1273 (in July)
- The University of Oxford and AstraZeneca’s AZD1222 (in August)
- Pfizer and BioNTech's BNT162 (in September)
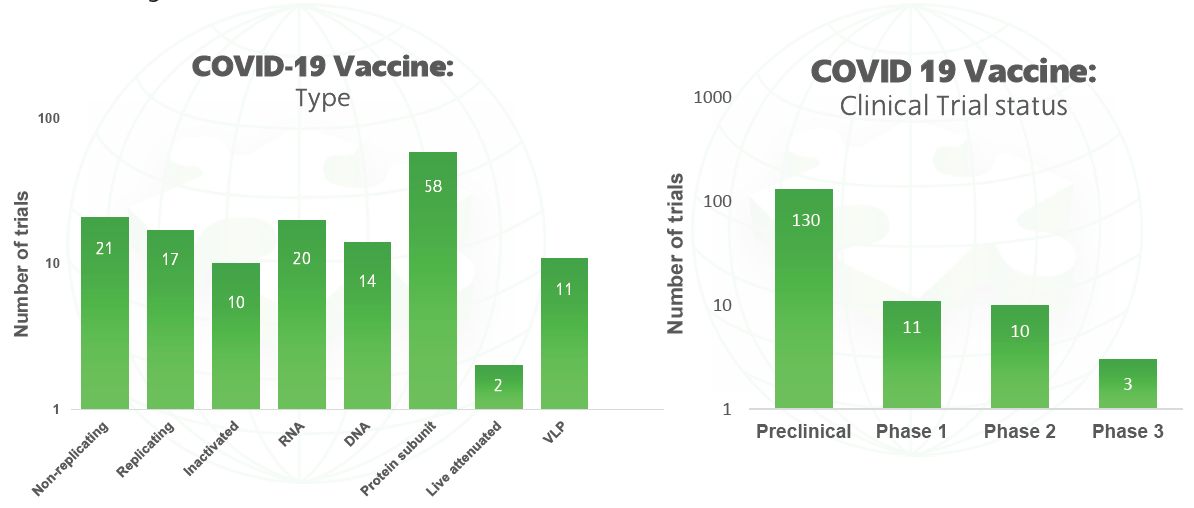
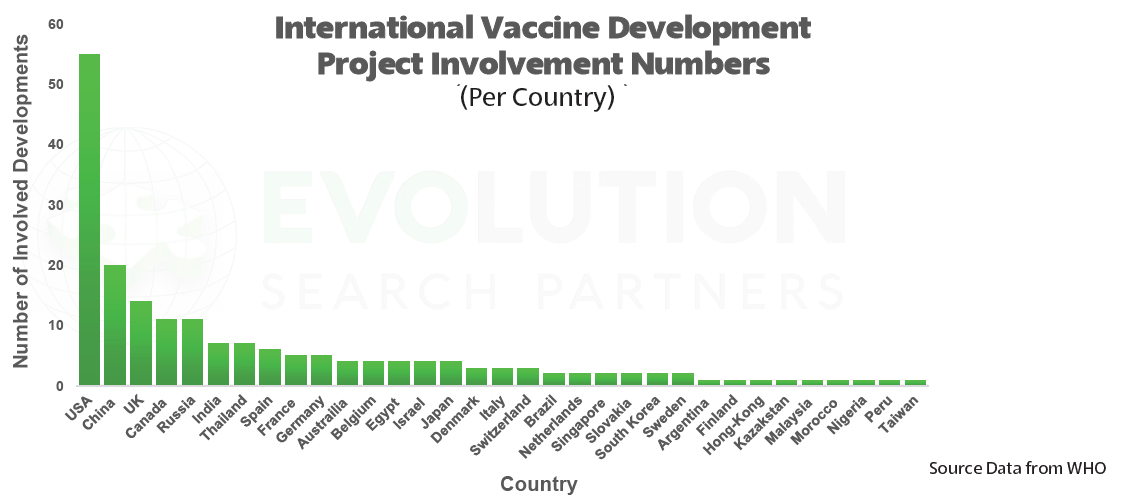
Since the virus first emerged in January, around 170 vaccine candidates are now in development, with 15 already in human trials. This briefing aims to highlight selected COVID-19 vaccine candidates currently in Phase I-III trials, as well as major candidates in pre-clinical stages of development and research.