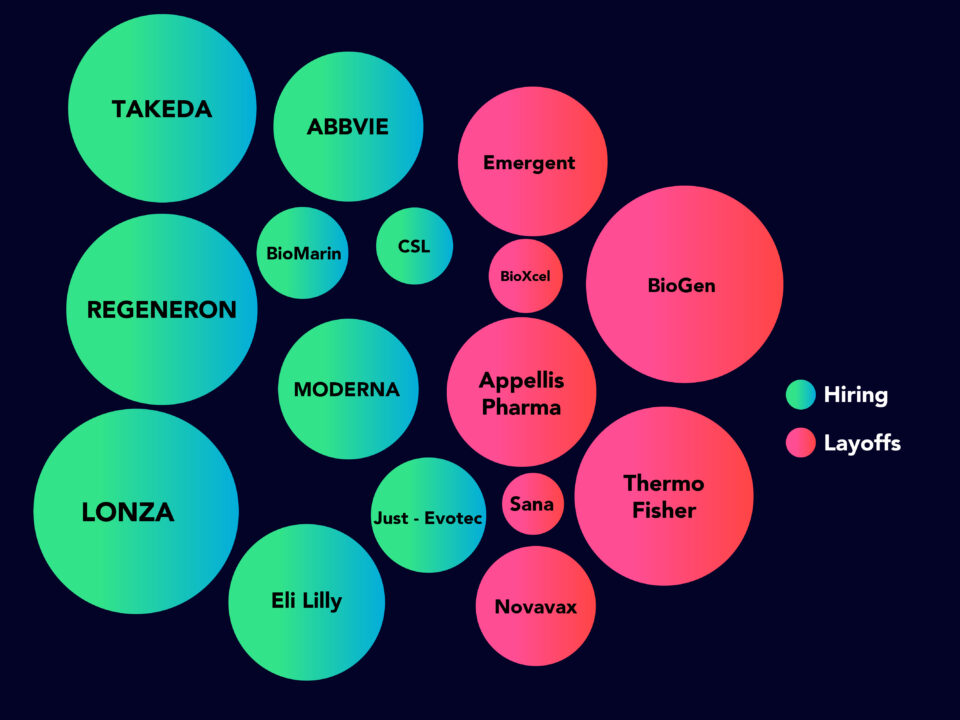
Assessment of COVID-19 Hiring Strategy May 2020
June 26, 2020
Evolution Executive News: Avid Bioservices Announce New President & CEO
June 30, 2020Moderna’s vaccine looks set to become the first vaccine to enter Phase III trials - the final phase before potential FDA approval.
The company announced that it would begin US trials in July and would conduct these trials alongside the National Institute of Allergy and Infectious Diseases (NIAID). It will include 30,000 participants, randomised and placebo controlled. Based on results from Phase I study, the 100 μg dose level was chosen as the optimal dosage, allowing for the maximum immune response whilst minimizing adverse reactions. Moderna says it can deliver approximately 500 million doses per year. Through a strategic collaboration with Lonza, Moderna will be able to manufacture up to 1 billion doses per year and they plan to start manufacturing even before the vaccine's efficacy is established, in an effort to get ahead of the competition.
Vaccine type
Messenger RNA: Genetic instructions for the coronavirus spike protein are encoded in mRNA, delivered via lipid nanoparticle.
Target supply
With Lonza, 1 billion doses per year.
External funding
Up to $483 million.
About mRNA-1273
mRNA-1273 is an mRNA vaccine against SARS-CoV-2 encoding for a stabilised form of the Spike (S) protein, which was selected by Moderna in collaboration with investigators from Vaccine Research Center (VRC) at the National Institute of Allergy and Infectious Diseases (NIAID), a part of the NIH.
The potential advantages of an mRNA approach to prophylactic vaccines include the ability to combine multiple mRNAs into a single vaccine, rapid discovery to respond to emerging pandemic threats and manufacturing agility derived from the platform nature of mRNA vaccine design and production.


Details
- Moderna's experimental coronavirus vaccine using messenger RNA technology, an unproven approach that instructs cells to produce specific proteins, can be used to make a vaccine much faster than traditional methods.
- The vaccine went from a computer design in January to human study in just three months. Since then, Moderna has kept up its record pace. Phase I data came in late May, as did the start of a midstage trial and a final study could begin in July, making the company's efforts one the best hopes for a vaccine potentially available within the aggressive timeframes of 'Operation Warp Speed.'
- No mRNA vaccine has been proven to prevent disease, nor manufactured and distributed at scale, let alone during a pandemic. Moderna still needs to show it can produce enough doses to vaccinate millions of people.